PROJECTS and FUNDING
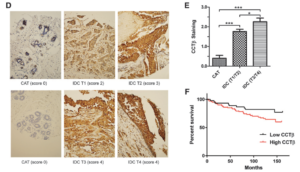
- New treatment and target for cancer. Cancer kills when cancerous cells invade vital organs like the brain or lungs. A curative approach, designed to attack a patient’s unique cancer, is needed to improve patient survival. To this end, we discovered a new anti-cancer agent called CT20p. CT20p is a small peptide formed by 20 amino acids, which are biological building blocks. CT20p prevents a cancer cell from attaching to tissues and, as a result, cancer cells lose needed survival signals and die. To understand how CT20p works, we found that the peptide specifically inhibits a protein called called chaperonin-containing-TCP1 or CCT, which is basically a helper protein that shapes other proteins into their final three-dimensional forms. Without CCT, a cancer cell cannot produce the components that it needs to move, grow and survive. Importantly, we found that cancers cells made more CCT than normal cells (see image below from Bassiouni et, 2016, CCR), and patients whose tumors contain large amounts of CCT, died more quickly. These findings suggest that inhibiting CCT with CT20p is an effective therapeutic approach that could improve the survival of cancer patients whose tumors contain CCT. The aims of our project are, first, to learn how CCT helps lung, prostate or breast cells become cancerous, and, second, to discover how CT20p binds and inhibits the activity of CCT to develop a screening assay that will help us find new CCT inhibitors. By completing the work proposed, we will produce a novel therapeutic designed to inhibit the invasive nature of breast, lung and prostate cancer with minimal side effects.
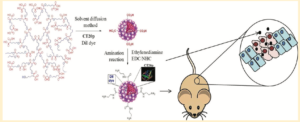
- Using nanotechnology to deliver a therapeutic peptide. Death due to prostate, lung or breast cancer occurs when the disease spreads to vital organs like the lungs or brain. Cancers that invades other organs cannot be cured, and patients with this complication may die within 5 years. Treatments for prostate, lung or breast cancer, such as chemotherapy and radiation, can also cause serious side effects, such as nausea and vomiting or lowered resistance to infections. To help men and women with life-threatening forms of cancer, new targeted agents are needed that deliver toxic drugs to the cancer cells, without damaging normal, healthy cells. One promising approach is to load cancer drugs into ultrafine particles or nanoparticles that can carry drugs safely through the body, delivering their drug cargo to tumors (see image below from Boohaker et al, 2012, Mol Pharm). The problem is that sometimes nanoparticles accumulate in other organs, like the liver, and fewer drugs are then delivered to tumors. One way to solve this problem is to “address” the nanoparticles by adding a molecule on their surface that only binds to cancer cells. In this project, we are developing nanoparticles that localize to prostate and breast cancer cells dependent on an important nutrient called folate. Cancer cells need folate to power their metabolism and may take up nanoparticles coated with folate more readily than normal cells. By delivering drugs using nanoparticles that are designed for enhanced circulation, we increase the amount of drug that reaches tumors, while decreasing uptake by healthy tissues. This work will yield a new drug delivery agent that could extend the life expectancy of cancer patients, while minimizing side effects.
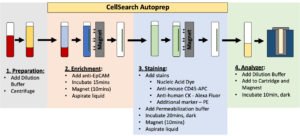
- One way to save lives lost to cancer is early diagnosis. The problem is that the symptoms of cancer are indeterminate and individuals may delay seeing their doctor until it is too late. About 10 years, a new way to detect cancer by counting the number of circulating tumor cells (CTCs) in the blood, called the CellSearch test, was developed. When the test detects more than three- five CTCs in a few teaspoons of blood, doctors know that the cancer is worsening (see imaging below). The test detects CTCs with “generic” markers found on most tumors. However, these markers may not be found on all types of tumors. Our project involves developing a new scheme to capture and count CTCs from blood. We rationalize that for CTCs to leave the primary tumor, new proteins are needed to give them an advantage. We hypothesize that CTCs upregulate a protein-folding complex called a chaperonin, which can be used for their detection. The proposed test for screening of CTCs from blood could help high risk individuals and also enable the subsequent acquisition of genome-wide data on these rare CTCs to better understand their origins and develop new targets for therapeutic intervention.